Corporate Emergency Response Measures
Corporate Emergency Response Measures
In order to deal with unexpected events, we have established risk management measures to govern evaluations of emergencies with larger potential impacts and formulation of emergency response measures. Our manufacturing plants also have risk management strategies that allow them to respond immediately and effectively to emergencies and accidents, and rigorous control and handling measures are implemented to address exigencies and reduce personnel injury and property loss.
These risk management strategies encompass safety, environmental protection, sanitation, fire protection, and security, as well as the establishment of an emergency reporting hotline that can be used to directly request assistance from the response units of the industrial park and local governments, thereby minimizing damages when emergencies or accidents occur and allowing for stable corporate operations.
Management and Response Measures for Business Interruptions
Bora Pharmaceuticals has instituted a Business Continuity Management (BCM)mechanism since 2020, encompassing reviews of all factors directly or indirectly affecting supply chains and evaluations of risk levels. We have also established a Business Continuity Team which is convened by our General Manager, and respective department heads are responsible for coordinating relevant factors:
- Raw material supply: The quality control and procurement departments conduct risk evaluations for each raw material supplier and establish alternative procurement plans for high-risk suppliers.
- Production equipment: The engineering, quality control, and manufacturing departments examine all production equipment, inventory spare parts, and ascertain parts supply and service interruption risks of all suppliers; changes to production processes or addition of new equipment may be necessary to prevent production interruptions.
- Personnel deployments should be fully reviewed to ensure reasonable assignment of production personnel and confirm that production personnel are equipped with the skills to provide support for each other so that production processes are not disrupted.
To ensure that production plants can take immediate action to address risks during emergency situations and minimize potential impacts from disasters, each production plant has established an individual emergency response plan that covers various emergencies and accidents. A commander in chief is responsible for deploying resources and bringing together all department heads to form a core command center. Additionally, we have also established fire protection teams composed of at least two trained personnel from each department who can quickly resolve emergency incidents including but not limited to fires, releases of hazardous energies, natural disasters, and life-threatening diseases or injuries.
According to our Environmental Safety and Health Emergency Response Plan, our General Manager serves as the administrator and person in charge of emergency response and evacuation plans and is responsible for establishing and formulating detailed emergency response and evacuation plans, and arranging evacuation measures for each area, in the event of accidents. The engineering and environmental safety and health departments are responsible for identifying the most appropriate and safe evacuation routes, and various managers and directors are responsible for conducting a headcount of personnel and ensuring safe evacuations for the entire plant until supporting personnel arrive at the designated assembly points to provide assistance.
Managers and directors work routinely with employees to review emergency procedures, emergency exit routes, and emergency evacuation maps posted in workplaces, and the Environmental Safety and Health Department works with local public emergency response measure providers to refine and update this plan. If production plants encounter any moderate to major events that require external assistance, a hotline should be established so that support and assistance may be sought from the industrial park or relevant management authorities. Depending on differing jurisdictions, assistance may also be sought by directly contacting local governments.
Epidemic Risk Management
Upon the outbreak of the COVID-19 pandemic, Bora Pharmaceuticals immediately activated response mechanisms. Apart from requiring each member of staff to measure body temperatures, disinfecting alcohol was made available at all main entrances, guardrooms, lobbies, dining tables, and restrooms for visitors and staff to disinfect their hands. Protective membranes were placed on elevator buttons, and were cleaned daily and replaced weekly, while signage indicating the maximum number of occupants, as well as where they should stand, were placed on the floors of all elevators.
In addition, personnel dispersals, shift changes, and work from home (WFH) measures were implemented as soon as the Central Epidemic Command Center issued a Level 3 epidemic warning; these measures were relaxed in early July as the pandemic slowed, allowing staff to resume normal work routines. No staff members became infected during this time and there were no risks of operational disruptions. The efforts of all departments enabled the Company to weather the pandemic safely and return to Level 2 epidemic status.
According to the Business Continuity Plan for Epidemics or Pandemic Flu established at Bora Pharmaceuticals, the Environmental Safety and Health Department and Human Resources Department should proactively inform staff of infectious disease information issued by the Centers for Disease Control of the Ministry of Health and Welfare, including disease types and methods of spreading. Regular updates should be provided by email or other effective measures to monitor and update employee status, as well as provide any and all useful information. In addition, the Environmental Safety and Health Department and Human Resources Department should provide thermometers, face masks, other medical supplies, and empty sealable bags at all on-site clinics, and prepare protective suits, rubber gloves, and protective goggles in sufficient quantities for the use of staff charged with providing assistance to affected employees. The following items should also be propagated within the Company and brought to the attention of all departments:
- Encourage employees to maintain good hygiene habits in the workplace.
- When sneezing or coughing, paper napkins should be used, and cleanup should be conducted carefully
- Avoid sharing cups or other tableware
- Remind employees to use soap and water to wash their hands thoroughly under the following circumstances:
● Before and after preparing food
● After going to the toilet
● Before and after meals
● After coughing or sneezing
● After removing personal protective equipment (PPE)
Our Business Continuity Plan for Epidemics or Pandemic Flu responds to COVID-19 conditions and recommend that when outbreaks reach pandemic status (Red Level), key operational personnel should be divided into two segregated teams. If any employee displays fever or other infectious disease symptoms, the Environmental Safety and Health Department and Human Resources Department should be informed immediately, and local hospitals should be contacted to arrange transport for potentially infected employees. In addition, all employees that came in contact with potentially infected employees over the past 14 days should be notified, and affected areas should be disinfected.
In addition to these measures, Bora Pharmaceuticals provides personal protection packages to all staff and executives traveling for work during the pandemic, with the contents of the package comprising a protective suit, gloves, face masks, and disinfecting alcohol. Each plant site should further establish management policies to enhance disease prevention measures based on the following employee regulations:
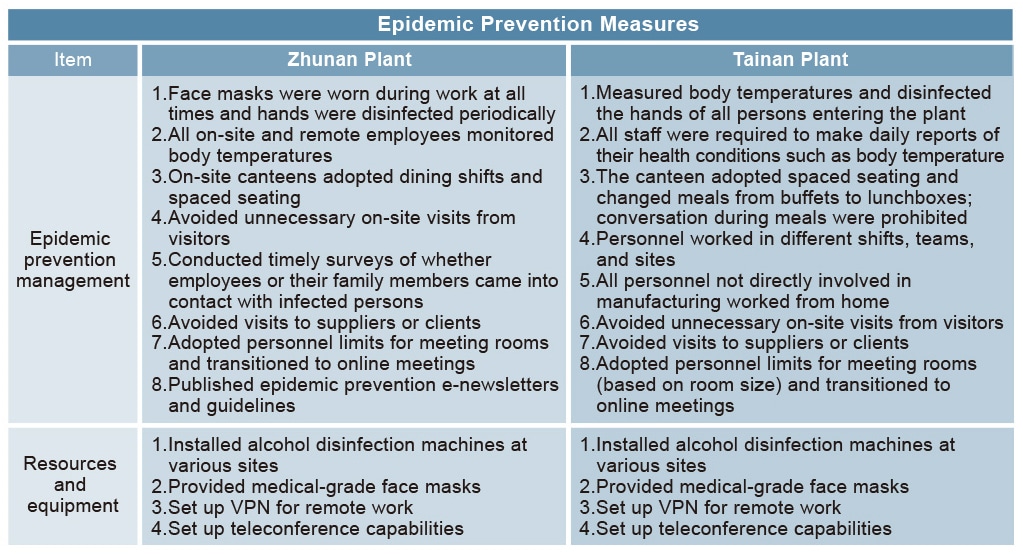
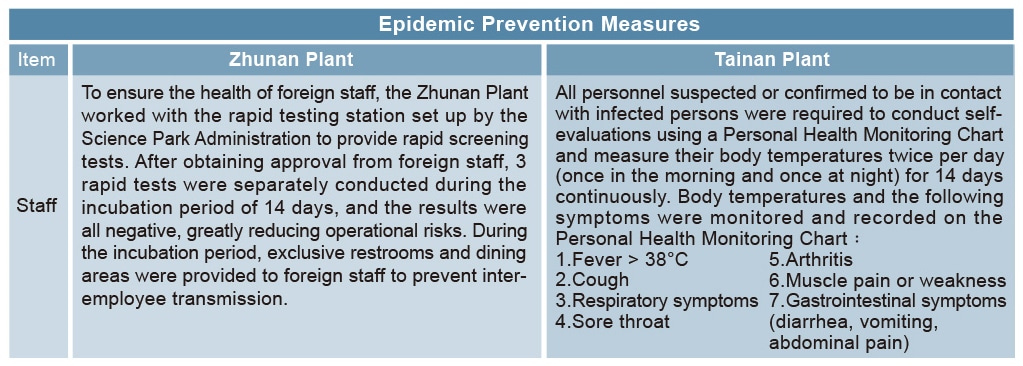
The following measures were adopted for higher risk personnel:
Epidemic Prevention Measures for Supplier On-site Visits
All visitors and vendors conducting necessary on-site visits were required to scan QR codes and fill in health proclamation forms when entering our sites, and plant security personnel measured body temperature and disinfected the hands of all visitors after the form was completed.

Response Measures to IT-Related Incidents

Management Measures for Manufacturing Units
The following standards were enacted for manufacturing units that were unable to fully switch to a remote working model:
- Partitions were installed in Manufacturing Department offices to prevent transmission through direct contact.
- During Level 3 epidemic situations, personnel were divided into different teams with separate day and noon shifts; personnel assigned to different shifts were required to avoid face-to-face conversation, and alcohol cleansing and disinfection procedures were conducted when leaving work areas.
- Personnel in formulation and packaging on-site areas were required to wear cleanroom face masks according to our Operational Clothing and Entering/Leaving Operational Areas Standard Operating Procedures, and we also required medical-grade face mask certified for epidemic prevention to be worn within cleanroom face masks, though N95 respirators could be worn alone for manufacturing processes requiring N95 respirators. We required all personnel to wear medical-grade face masks in secondary packaging areas at all times.
- Apart from rigorous prevention of cross-contamination between processes in manufacturing areas, we also prohibited gathering and chatting in public spaces.
- Formulation and packaging team meetings were temporarily cancelled, and important notices were instead distributed through LINE Groups. Original meeting periods were used to intensify environmental cleaning efforts.
