Legal Compliance
Mechanisms for Handling Regulatory Changes
Because regulatory knowledge is updated frequently, our Regulatory Department actively designates personnel to participate in regulatory training organized by the Taiwan Food and Drug Administration (TFDA) and the Center for Drug Evaluation (CDE), notifies departments affected by new regulations, and organizes internal training program.
In 2021, we participated in 40 regulatory training sessions organized by competent authorities and organized 4 internal cross-departmental training sessions. Announcements and information from competent authorities and public associations are conveyed to relevant departments by email as soon as possible. If products need to be changed, we conduct in-depth discussions with our quality departments to formulate strategic changes and schedules for submitting relevant documentation.
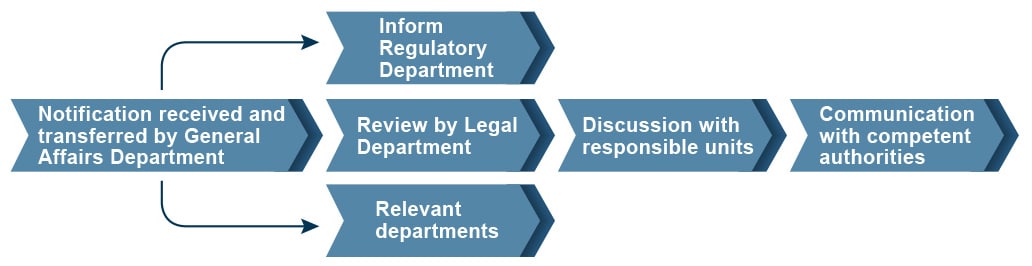
Mechanisms for Handling Illegal Incidents
Notifications of regulatory violations received by our General Affairs Department are transferred to our Legal Department, which then discusses the same with our Regulatory Department and relevant departments; personnel from the department which incurred the violation are responsible for explaining specific corporate actions and improvement measures to the competent authorities.