Management Approach
Bora Pharmaceuticals adheres to international quality standards and regulations, establishing comprehensive control systems that span the entire drug lifecycle-from R&D to manufacturing, testing, and supply chain management. Safety management plans and reporting mechanisms are in place to ensure product quality and safety. By continuously enhancing SOPs and compliance practices, Bora is committed to protecting patient health, driving improvement, and pursuing excellence.
Regulatory Compliance and Audit System
A. Facility-Specific Regulatory Compliance and Audits
- Facility-Specific Regulatory Compliance and Audits
Each Bora facility complies with regulations based on its product types and markets. Sites undergo regular audits by regulatory authorities and customers.The following regulations apply:
Standards:
- PIC/S GMP
- EU GMP
- US FDA 21 CFR
Regulatory Authorities:
- US FDA (United States)
- EMA (European Union)
- Health Canada (Canada)
- PMDA (Japan)
- Compliance with the Latest Regulations
- Bora subscribes to the “Regulatory Intelligence Newsletter” to stay updated on the latest regulatory changes and FDA inspection outcomes.
- This information is distributed to each facility monthly, with necessary actions taken where needed.
- Quarterly meetings are held to promote awareness and timely responses to regulatory changes.
B. Self-Inspections
- Audit Mechanism
- Bora conducts independent self-audits to assess the effectiveness and compliance of its quality systems.
- Each quality system is audited at least once a year per the audit plan.
- Bora conducts independent self-audits to assess the effectiveness and compliance of its quality systems.
- Continuous Improvement
- The results of self-audits are utilized to ensure continuous improvement and compliance, enhancing the effectiveness of the quality management system.
- Audit Mechanism
Introduction to Standards Followed by Taiwan Facilities
| Facility | Inspection Year/Month | Standard Followed | Certification Result |
|---|---|---|---|
| Zhubei Facility | Mar-2023 |
| Passed, awarded a 2.4-year certification |
| Zhunan Facility | Dec-2022 |
| Passed, awarded a 3.5-year certification |
| Jun-2019 |
| Passed, awarded a 3.5-year certification | |
| May-2019 |
| Passed, with zero deficiencies | |
| Feb-2018 |
| Passed | |
| Tainan Facility | Aug-2020 |
| Passed |
| Zhongli Facility | Nov-2023 |
| Passed |
| Jul-2023 |
| Passed | |
| Jul-2021 |
| Passed | |
| Dec-2020 |
| Passed | |
| Aug-2020 |
| Passed | |
| Feb-2020 |
| Passed | |
| Aug-2019 |
| Passed | |
| Jul-2018 |
| Passed | |
| May-2018 |
| Passed | |
| Taoyuan Facility | Dec-2022 |
| Passed |
| Dec-2021 |
| Passed, awarded a 3.5-year certification |
Introduction to Standards Followed by Overseas Facilities
| Plant | Inspection Year/Month | Standard Followed | Certification Result |
| Canada Facility | Jun-2025 | Japanese Ordinance 2021 (PDMA) | Passed |
| Feb-2024 | Heath Canada GMP guidance | Passed | |
| Minnesota and Plymouth Facilities, USA | Jul-2024 | US FDA 21CFR (CDER PAI) | Passed |
| Maryland Facility, USA | Sep-2024 | PIC/S GMP (Turkish MOH) | Passed |
| Jul-2024 | CBER GMP Compliance Program Guide CP7345.848, Inspection of Biological Drug Products (PAC 42848F – Level 1 GMP Inspection – Plasma Derivates) | Passed |
Quality Management System
A. Product quality management
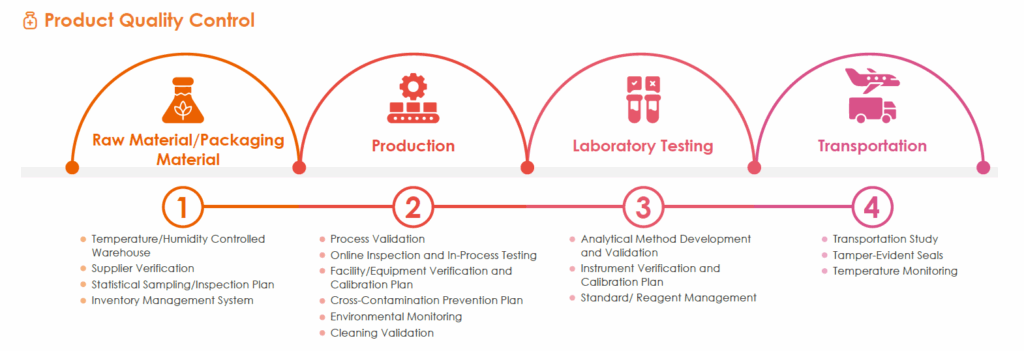
- Quality is managed at every stage- from drug production to shipmen- through defined controls that ensure consistent product standards.
B. Quality System Monitoring
- Monitoring Mechanism
- The Quality Unit analyzes the effectiveness of the quality system on a monthly basis and reports to senior management.
- Senior management actively promotes a production environment that complies with regulatory requirements.
- Quality Committee Operations
- The Quality Committee, organized by senior management, holds monthly meetings.
- It reviews, addresses, and improves quality issues arising in various facilities.
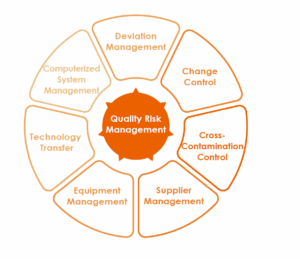
C. Quality Risk Management
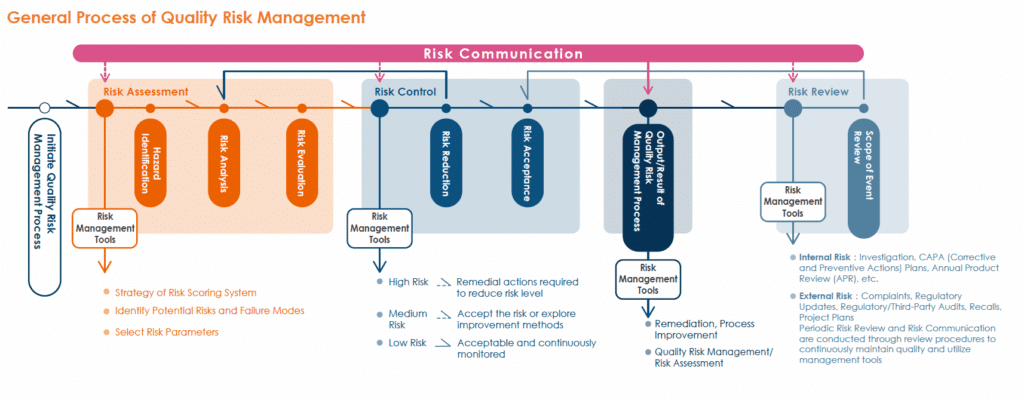
- Risk Assessment and Management
- Quality risk management is conducted in accordance with the ICH Q9 Quality Risk Management guidelines to assess product quality risks.
- Risk management tools are applied to identify, analyze, evaluate, control, communicate, and review potential risks related to processes and quality systems.
- Timely Decision-Making and Regulatory Compliance
- Through risk management, Bora is able to make optimal decisions in real time, proactively ensuring compliance with regulatory requirements.
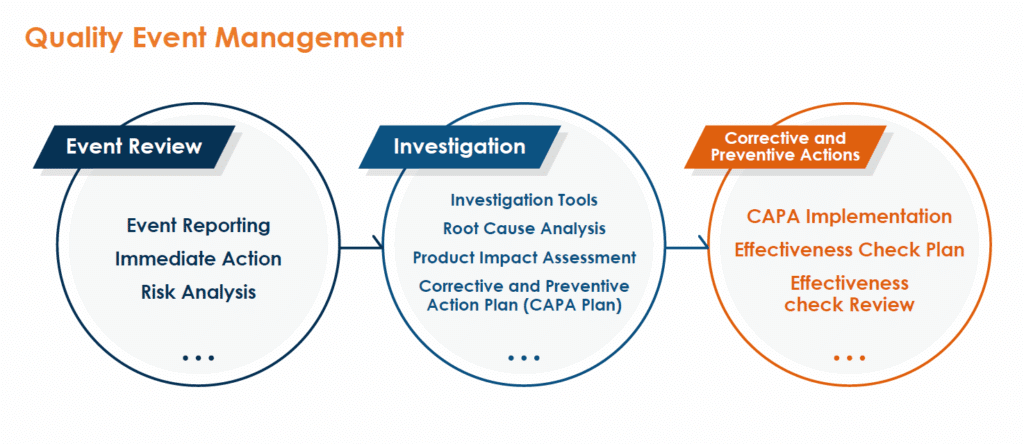
Management of Quality event
A. Investigation and CAPA
- Bora has a comprehensive deviation investigation management system. The process includes identifying deviations, determining root causes, and implementing corrective and preventive actions (CAPA) to prevent recurrence.
B. Critical Quality Event and product recall
- When a quality event is identified, the Quality Unit will assess whether it is a critical incident and notify the drug license holder within one business day. Customers will decide whether a product requires market warning, recall, withdrawal from the market, or stock recovery based on the information provided by Bora.
- The drug license holder is responsible for coordinating and approving the recall process and communicating with government authorities/agencies. If needed Bora’s QA team conducts product traceability checks, gathers data, and leads investigations.
- In addition, Bora executes material traceability checks at least once per year.